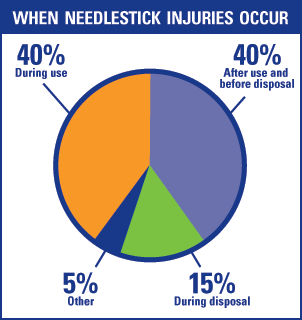
Needlestick injury is a serious problem among healthcare workers.
It is estimated that 800,000 needlesticks occur each year in the US, with about 2% of these likely to be contaminated with HIV. The risk of infection with Hepatitis B (HBV) or Hepatitis C (HCV) from a needlestick injury is far greater than the risk of contracting HIV however (2% – 40% for HBV and 3% – 10% for HCV). This is due in part to the fact that the prevalence of HIV in the general population is lower than that of Hepatitis B or C. It is also due to the fact that a greater exposure to viral load is required to contract HIV than either HBV or HCV. While these are by no means the only infections that can be transmitted via a needlestick, they are among the most serious and all have the potential to cause death.
As one would expect, nurses and phlebotomists have the greatest incidence of needlestick injury.
Since the passing of the Bloodborne Pathogens Standard in 1991 and its revision in 2000, much more emphasis has been placed on preventing these types of injuries in the healthcare setting. Employers have the responsibility of protecting their employees and are required by law to institute policies and provide equipment that aids in this goal.
Engineering controls (safety devices), work practice controls (prohibiting recapping of needles, disposal of sharps immediately after use, having sharps containers close to the work area, etc.), and developing a comprehensive Exposure Control Policy have helped reduce the number of needlestick injuries each year, but there is still much room for improvement.
OSHA has mandated the use of safety devices, but they have not specified which devices must be used. They do require employers to have at-risk staff for exposure evaluate all safety devices available in order to determine which are most effective, and these must be provided for employees to use.
Hepatitis B vaccine must also be made available free of charge for all employees with risk of exposure…
…as well as free follow-up care post exposure to bloodborne pathogens for any employee with a needlestick injury. Having a comprehensive Exposure Control Policy in place and requiring all employees be trained in its procedures is one of the most effective methods of reducing or eliminating needlestick injury.
Not all needlestick injuries are preventable, but the majority of these injuries can be prevented by using safer needle devices.
Properly designed devices should include all the following features:
- Provide a barrier between the hands and the needle after use;
- Allow or require the worker’s hands to remain behind the needle at all times;
- Have safety features integral to the device itself rather than as accessories;
- Be in effect before disassembly and remain in effect after disposal to protect downstream workers;
- Be simple and easy to operate with little or no training.
- Passive safety devices (features remain in effect before, during, and after use without the worker having to activate them) are preferable to active devices, which require the worker to activate a safety mechanism and leave the worker at risk if this is not done.
- http://www.tdict.org is a link to guidance and forms for evaluating all types of safety devices.
What should be done if a needlestick injury occurs?
First, encourage bleeding at the site of injury and wash the site with soap and water (flush exposure to nose, mouth, or irrigate eyes with saline). The injury must be reported (determine the proper mechanisms within your practice) and medical advice must be sought – practices may choose to use a local occupational medicine practice for testing and treatment.
Both the source patient and the injured person should be checked for HIV status and for HBV and HCV. If the source patient tests positive for HIV, prophylactic medication should be begun immediately. Follow-up testing should be done on the injured person at 6 weeks and 4-6 months for HCV, and at 6 weeks, 3, 6, and 12 months for HIV. HBV has become less of an issue with the requirement that all healthcare workers be offered Hepatitis B vaccine, but if the worker declined HBV vaccination when it was offered, post exposure monitoring should include HBV.
The work environment should be studied prior to writing an Exposure Control Plan to determine which areas provide the most risk and which workers are subject to potential exposure. The Plan should then be created to help eliminate as many risks as possible, with emphasis placed on ensuring all staff at risk are thoroughly trained in its implementation. Once an exposure has occurred, the root cause should be determined and corrective action taken to prevent its recurrence, whether that means amending the Exposure Control Plan or retraining the staff or both.
You can download a sample Exposure Control Plan from OSHA here.
Consultant Elizabeth “Libby” Knollmeyer, B.S., MT (ASCP) has over 40 years experience in the laboratory industry and has set up many laboratories! She specializes in financial, operational management and compliance issues for both hospital and physician office laboratories. Libby has a wide variety of experience with her areas of special expertise including financial review and management, Quality Management protocols, outreach development, compliance and regulatory assistance, lab design and up fitting, lab remodeling, and market research for IVD manufacturers. She works independently and with large consulting groups to provide interim management for hospitals, and serves as adviser to lab equipment and supply distributors. She consults (and enjoys traveling) throughout the US and internationally. She can be reached at (336) 288-5823 or at eknollmeyer@triad.rr.com.